LENVIMA dosing and administration
Once a day. Every day. With or without food1
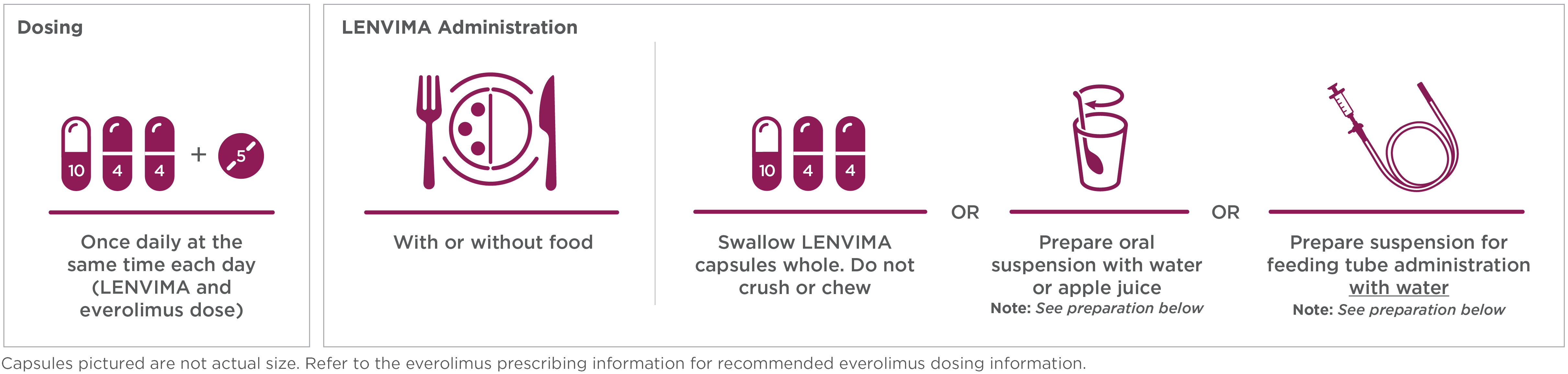
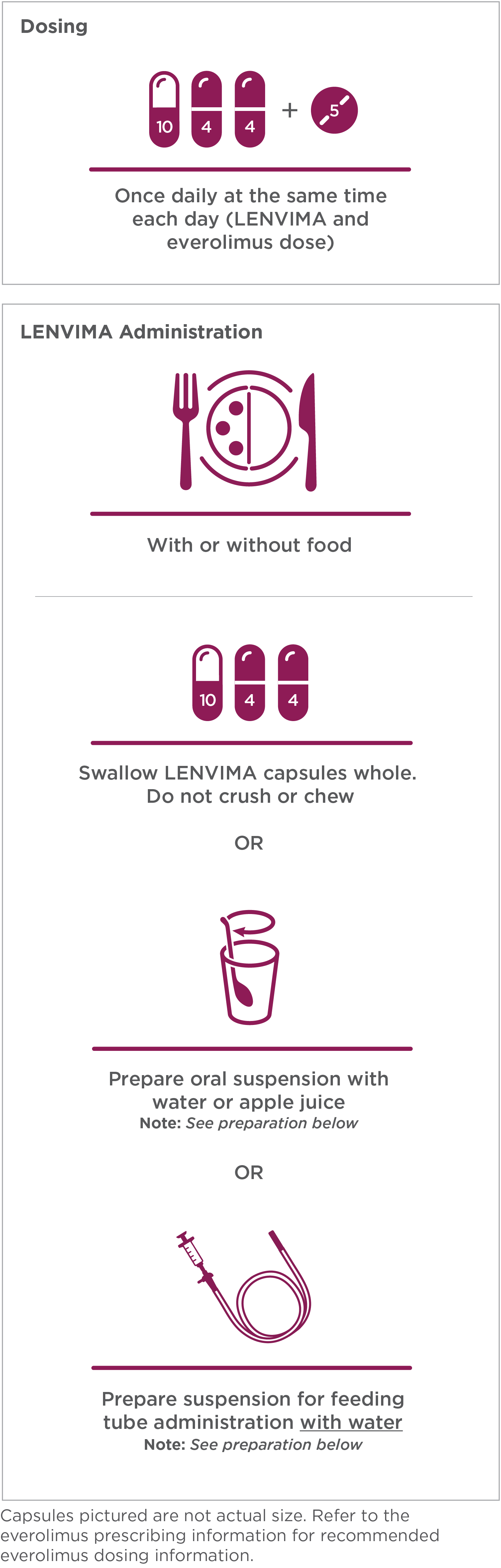
Recommended dose: 18 mg LENVIMA (one 10-mg capsule and two 4-mg capsules) + one 5-mg tablet of everolimus.1
- Continue LENVIMA until disease progression or unacceptable toxicity
- The approved combination contains half of the 10-mg dose that was used in the everolimus monotherapy arm of Study 205. The half-life of LENVIMA is approximately 28 hours
- If a patient misses a dose and it cannot be taken within 12 hours, then that dose should be skipped and the next dose should be taken at the usual time of administration
- Place the required number of capsules, up to a maximum of 5, in a small container (approximately 20 mL capacity) or syringe (20 mL). Do not break or crush capsules
- Add 3 mL of liquid to the container or syringe. Wait 10 minutes for the capsule shell (outer surface) to disintegrate, then stir or shake the mixture for 3 minutes until capsules are fully disintegrated and administer the entire contents
- Next, add an additional 2 mL of liquid to the container or syringe using a second syringe or dropper, swirl or shake and administer. Repeat this step at least once and until there is no visible residue to ensure all of the medication is taken
- If 6 capsules are required for a dose, follow these instructions using 3 capsules at a time
Preparation of suspension:
If LENVIMA suspension is not used at the time of preparation, LENVIMA suspension may be stored in a refrigerator at 36°F to 46°F (2°C to 8°C) for a maximum of 24 hours in a covered container. If not administered within 24 hours, the suspension should be discarded.
Note: Compatibility has been confirmed for polypropylene syringes and for feeding tubes of at least 5 French diameter (polyvinyl chloride or polyurethane tube) and at least 6 French diameter (silicone tube).
Everolimus is not distributed by Eisai Inc.
Dose adjustments for renal or hepatic impairment
Recommended dose of LENVIMA for renal or hepatic impairment1
- No dose adjustment is recommended in patients with mild or moderate renal or hepatic impairment. Patients with end-stage renal disease were not studied
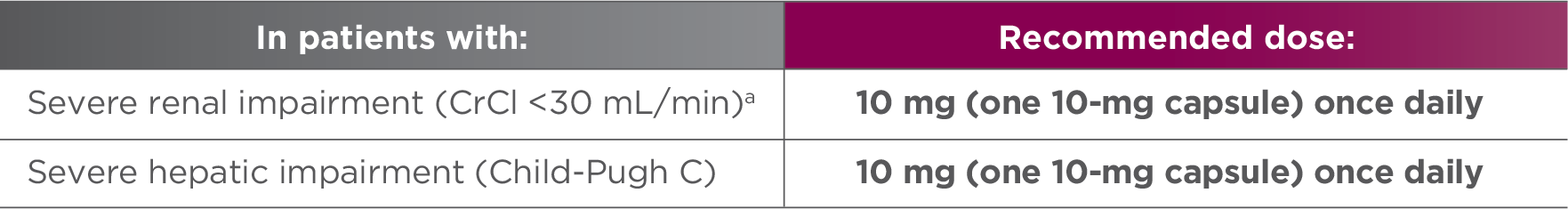
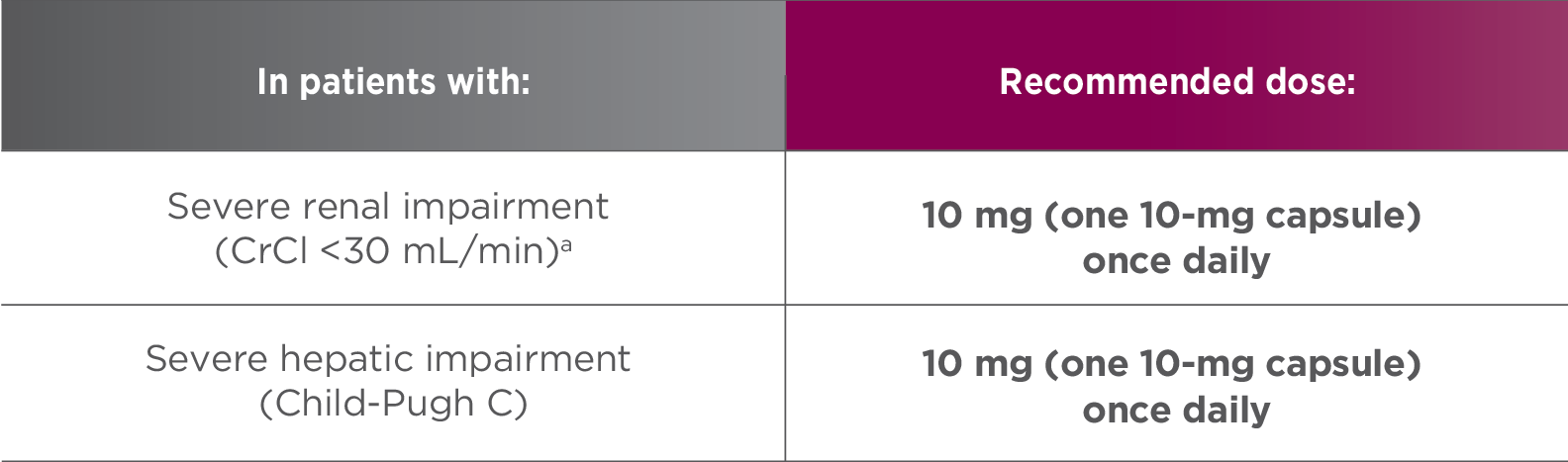
CrCl=creatinine clearance.
- aAs calculated by the Cockcroft-Gault equation.
You can modify dosing with the existing blister pack
Every pack of LENVIMA includes a 30-day treatment supply consisting of 6 individual blister cards. Each blister card contains a 5-day supply. Patients will receive a pack specific to their prescribed dose.
For example, if reducing the dose of LENVIMA from 18 mg to 14 mg, have your patient take one 10-mg capsule and one 4-mg capsule once daily. The recommended reduced dosages for aRCC are 14 mg, 10 mg, and 8 mg.
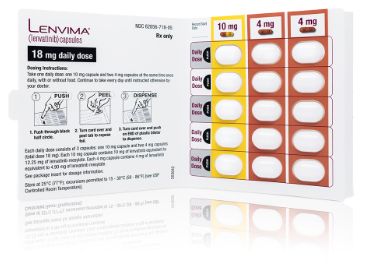
Two separate prescriptions are required for the combination
Everolimus is not distributed by Eisai Inc.
aRCC=advanced renal cell carcinoma.

Eisai Engage
Connect to HCP services at Eisai Engage to request materials for your practice, contact a sales representative, and more.
Visit Eisai Engage now