Adverse reactions observed in Study 205: LENVIMA + everolimus
Most common serious ARs (≥5%) in the LENVIMA + everolimus arm1
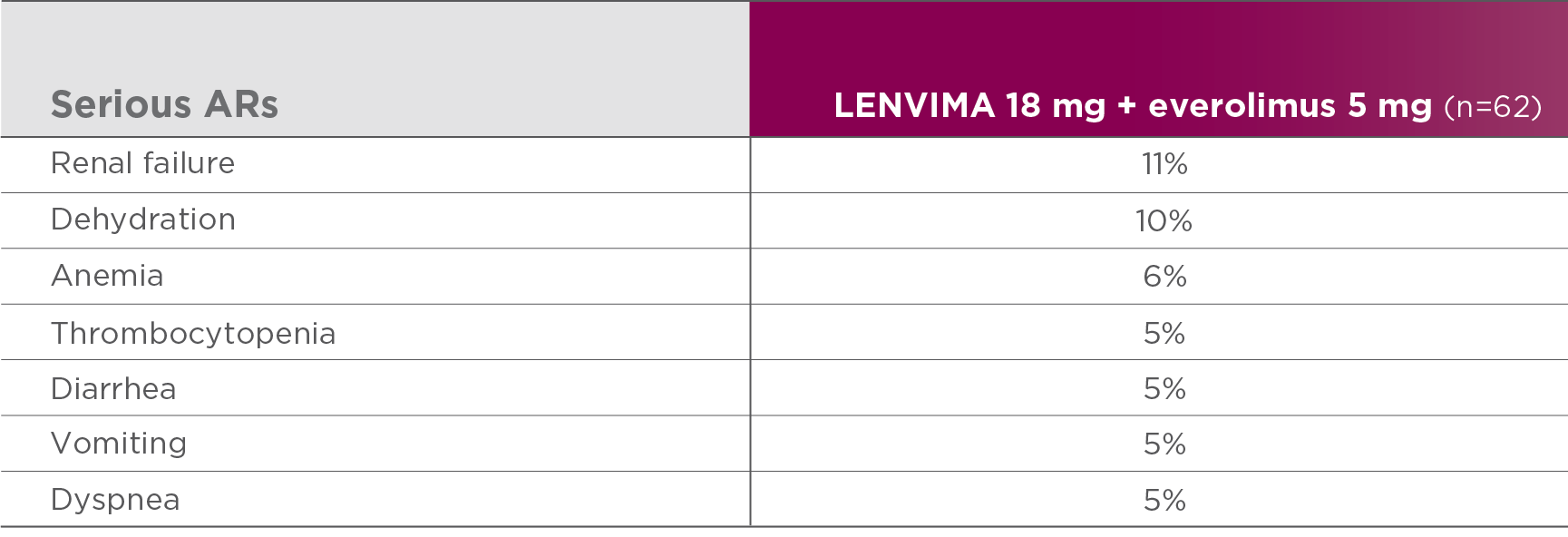
Study 205 was not designed to demonstrate a statistically significant difference in AR rates for LENVIMA in combination with everolimus, as compared to everolimus alone.1
AR=adverse reaction.
Most common grade 3-4 ARs (≥5%) in the LENVIMA + everolimus arm1
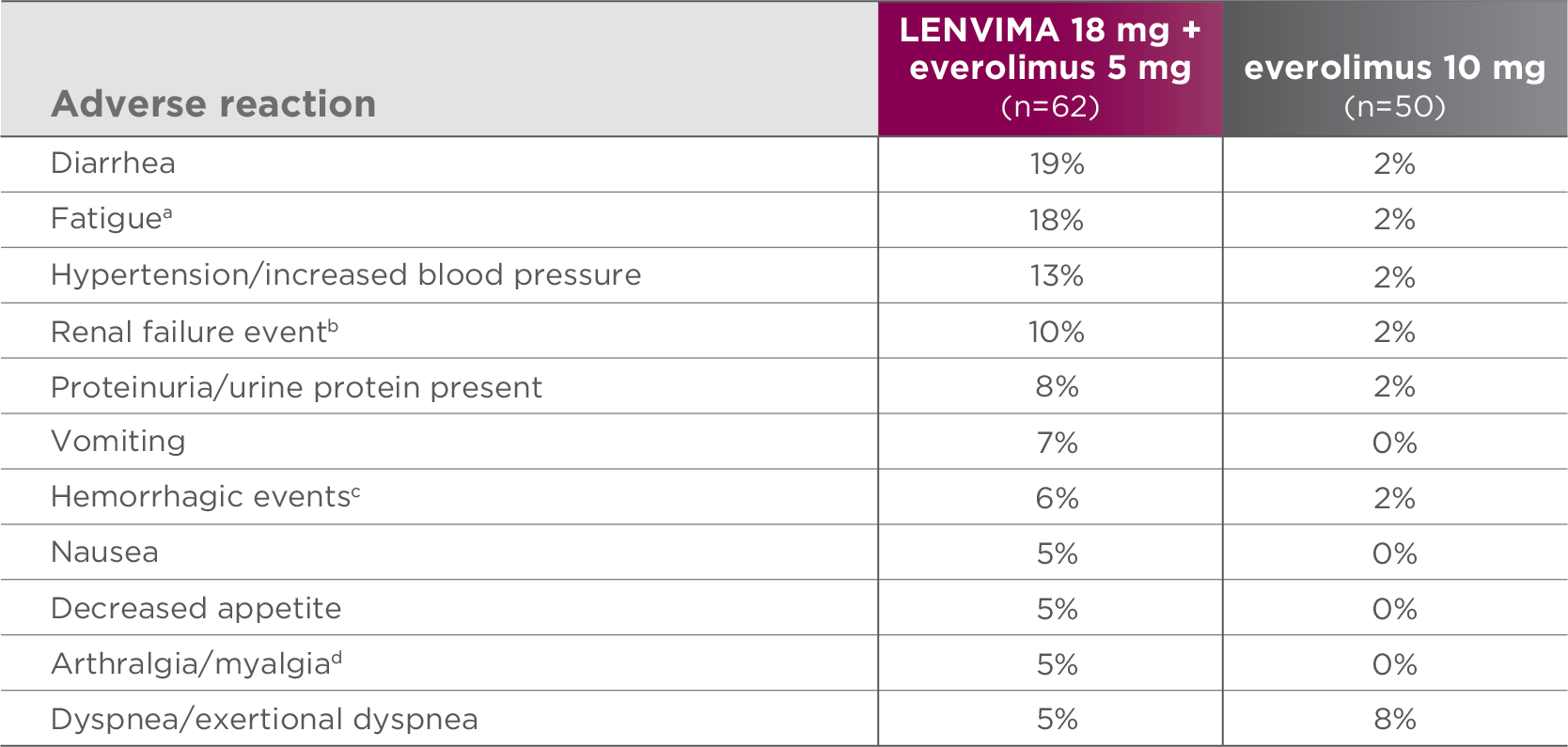
Study 205 was not designed to demonstrate a statistically significant difference in AR rates for LENVIMA in combination with everolimus, as compared to everolimus alone.1
AR=adverse reaction.
- aIncludes asthenia, fatigue, lethargy, and malaise.
- bIncludes blood creatinine increased, blood urea increased, creatinine renal clearance decreased, nephropathy toxic, renal failure, renal failure acute, and renal impairment.
- cIncludes hemorrhagic diarrhea, epistaxis, gastric hemorrhage, hemarthrosis, hematoma, hematuria, hemoptysis, lip hemorrhage, renal hematoma, and scrotal hematocele.
- dIncludes arthralgia, back pain, extremity pain, musculoskeletal pain, and myalgia.
Grade 1-4 ARs occurring in >15% of patients in the LENVIMA + everolimus arm1*
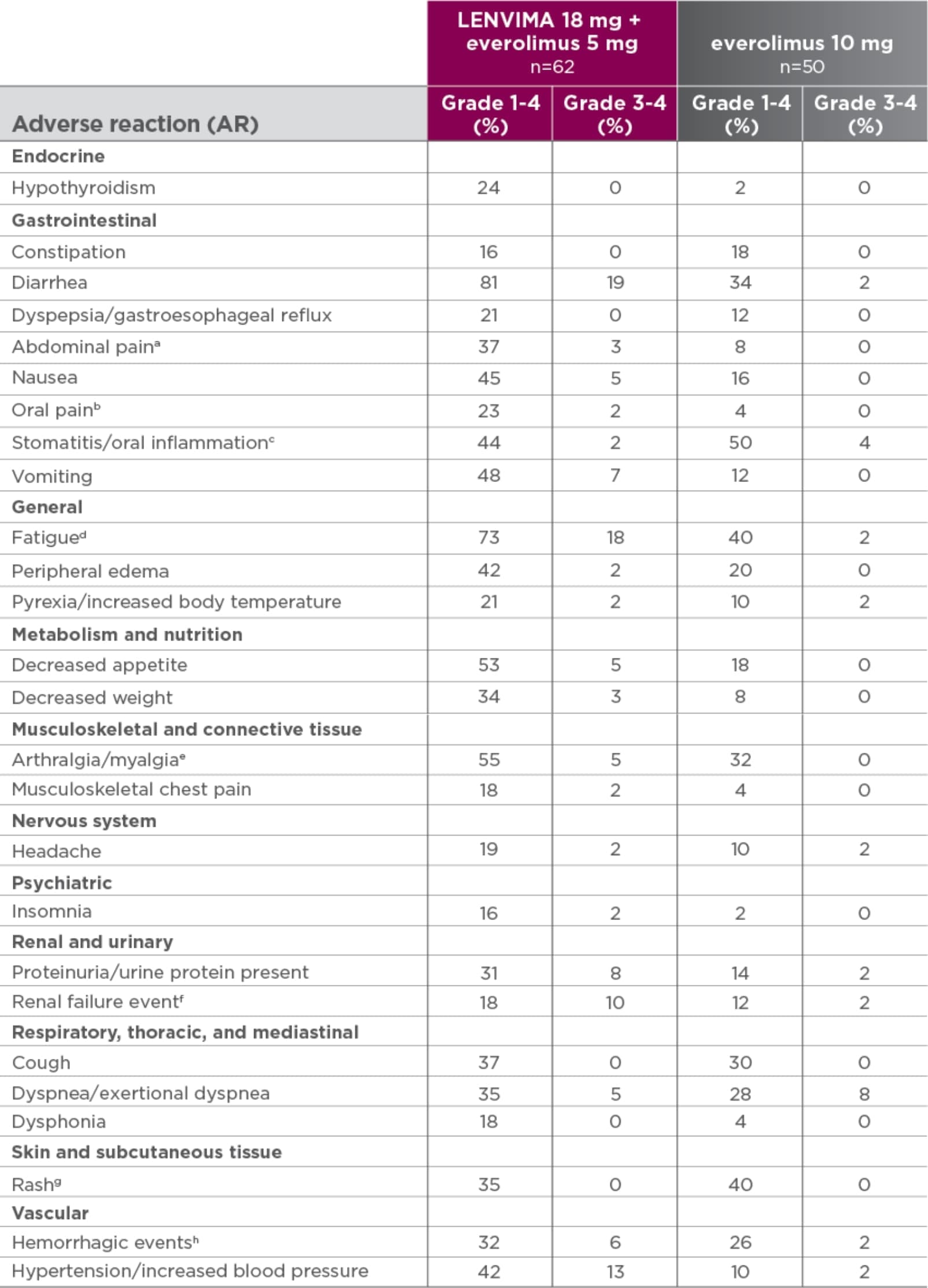
Study 205 was not designed to demonstrate a statistically significant difference in AR rates for LENVIMA in combination with everolimus, as compared to everolimus alone.1
- *The safety data are derived from Study 205 and an additional 11 patients in the dose escalation portion of the study who received LENVIMA 18 mg + everolimus 5 mg.
- aIncludes abdominal discomfort, gastrointestinal pain, lower abdominal pain, and upper abdominal pain.
- bIncludes gingival pain, glossodynia, and oropharyngeal pain.
- cIncludes aphthous stomatitis, gingival inflammation, glossitis, and mouth ulceration.
- dIncludes asthenia, fatigue, lethargy, and malaise.
- eIncludes arthralgia, back pain, extremity pain, musculoskeletal pain, and myalgia.
- fIncludes blood creatinine increased, blood urea increased, creatinine renal clearance decreased, nephropathy toxic, renal failure, renal failure acute, and renal impairment.
- gIncludes erythema, erythematous rash, genital rash, macular rash, maculopapular rash, papular rash, pruritic rash, pustular rash, and septic rash.
- hIncludes hemorrhagic diarrhea, epistaxis, gastric hemorrhage, hemarthrosis, hematoma, hematuria, hemoptysis, lip hemorrhage, renal hematoma, and scrotal hematocele.
Post hoc analysis of time to first onset of select ARs2
Identify points in treatment when select ARs emerged with LENVIMA + everolimus in Study 205
Median weeks; AR (n=62)*
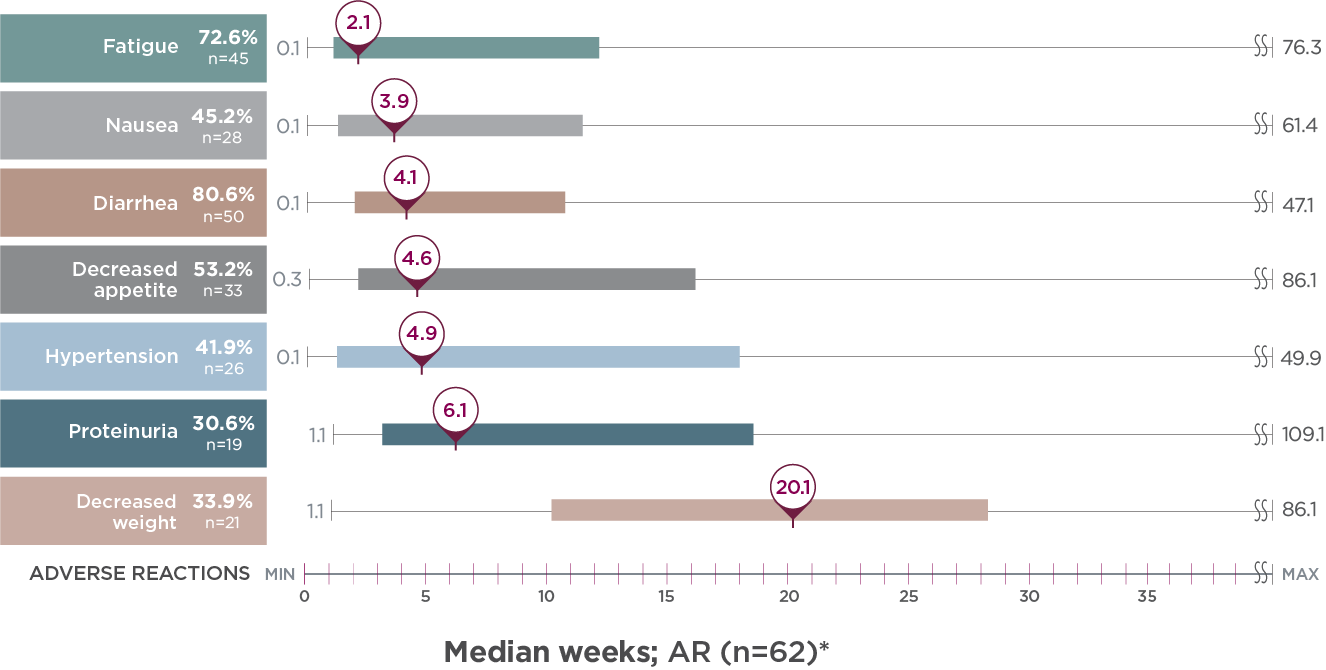
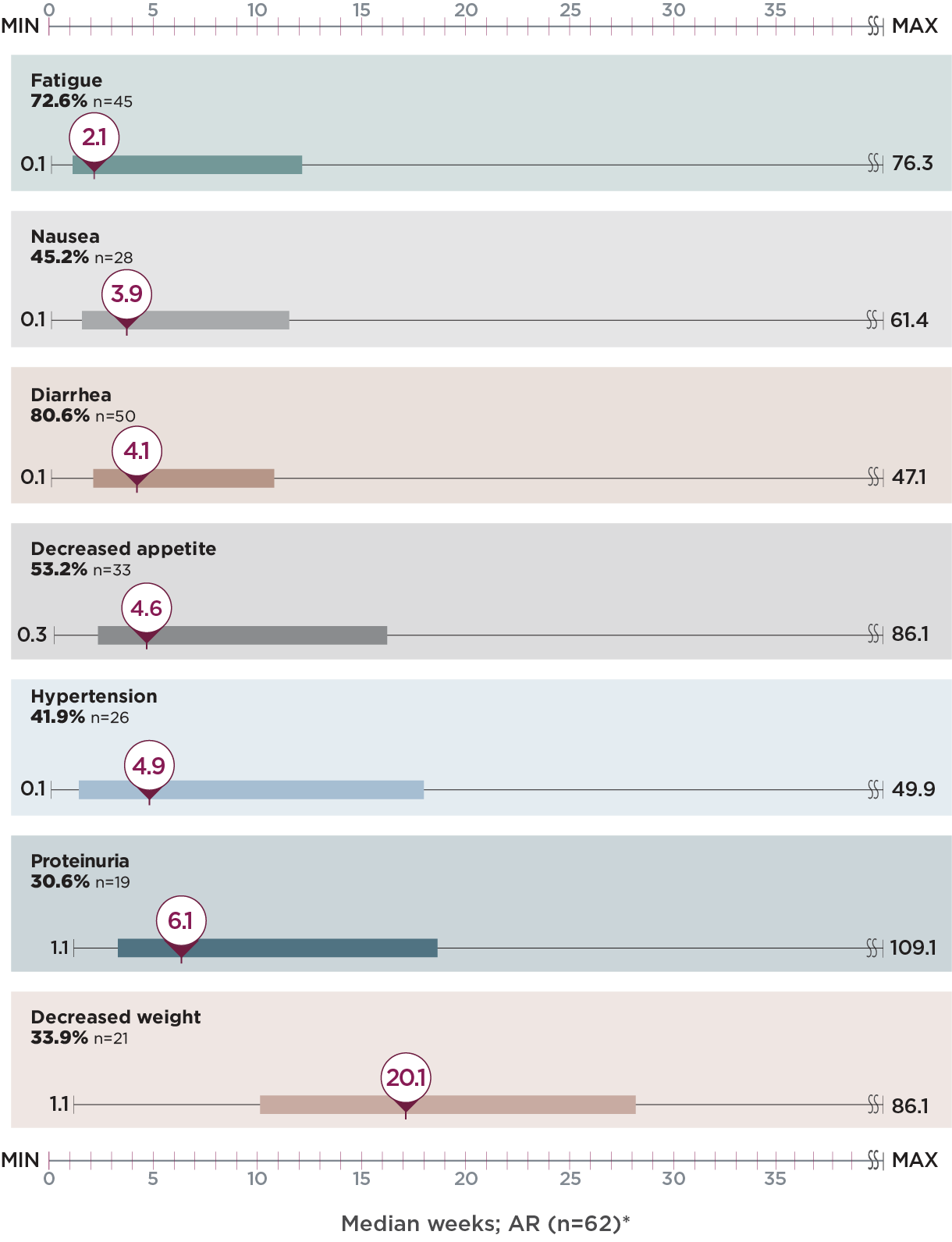
- Monitor your patients for ARs throughout treatment with LENVIMA + everolimus
Limitation: This is a post hoc exploratory analysis for descriptive purposes only; no conclusion can be drawn.
AR=adverse reaction.
*The bar represents the time to first onset of select ARs for the middle 50% of the patients who experienced that AR from quartile 1 to 3.
