The only NCCN Category 1 preferred first-line systemic therapy option


Lenvatinib (LENVIMA): the only NCCN Category 1 preferred first-line systemic therapy option by the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for the treatment of locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer (RAI-refractory DTC)*†‡
*Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Thyroid Carcinoma V.1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 28, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
†Category 1: Based upon high-level evidence (≥1 randomized phase 3 trials or high-quality, robust meta-analyses), there is uniform NCCN consensus (≥85% support of the Panel) that the intervention is appropriate.
‡Preferred intervention=interventions that are based on superior efficacy, safety, and evidence; and, when appropriate, affordability.
#1 prescribed 1L therapy for certain patients with RAI-R DTC§
§Source: Ipsos US Oncology Monitor (Mar 2024 to Feb 2025, 242 doctors in the US reporting on 1,577 Stage IV DTC patients seen in consultation, data collected online.) Data © Ipsos 2025, all rights reserved.
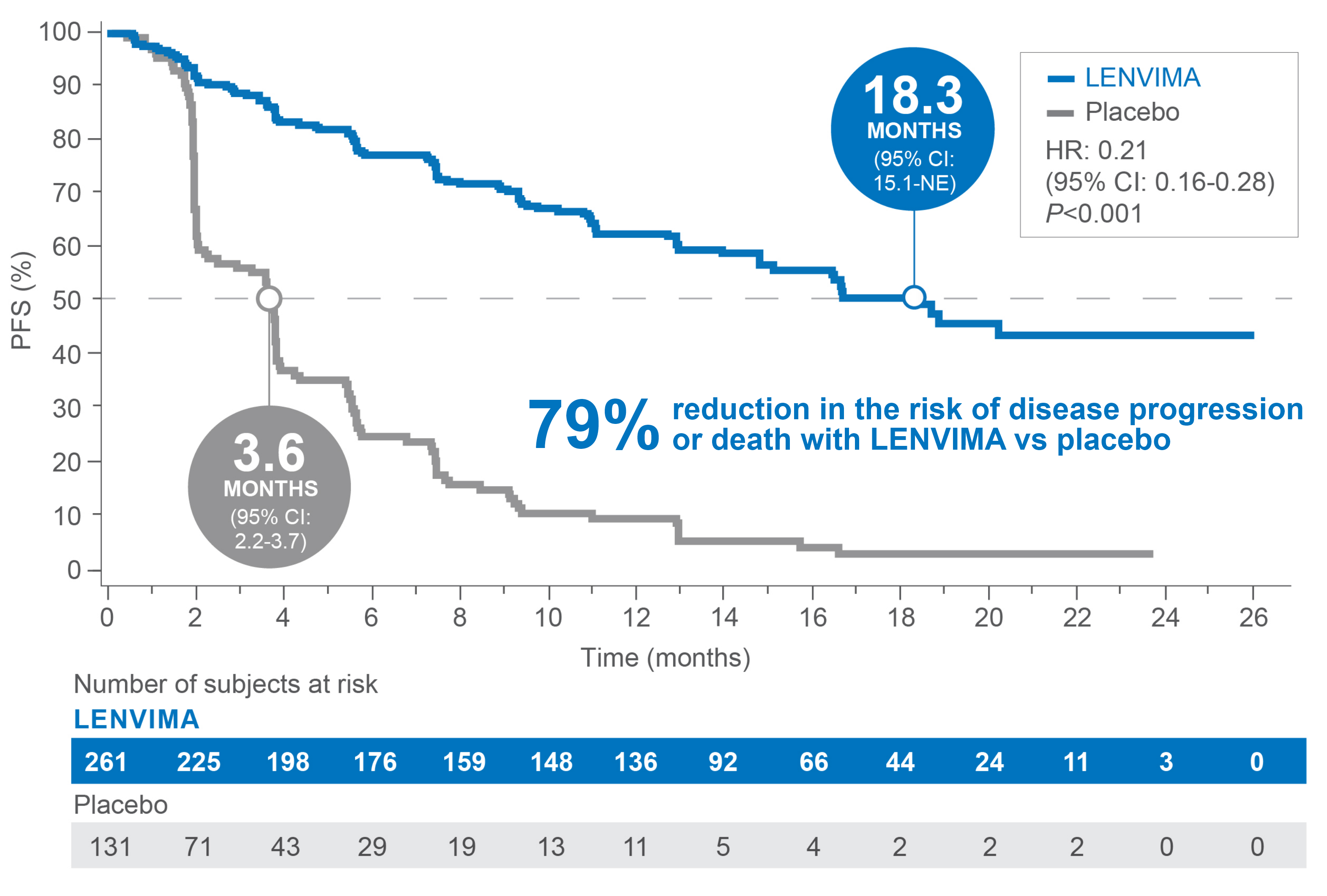
PRIMARY ENDPOINT
Superior PFS benefit
Median PFS: 18.3 months with LENVIMA vs 3.6 months with placebo1
• 18.3 months median PFS: (95% CI: 15.1-NE) with LENVIMA vs 3.6 months (95% CI: 2.2-3.7) with placebo (HR: 0.21 [95% CI: 0.16-0.28]; P<0.001)
SELECT study design
SELECT study results based on a phase 3, multicenter, randomized, double-blind, placebo-controlled trial in patients with locally recurrent or metastatic RAI-R DTC (N=392) who have had radiographic evidence of disease progression within 12 months prior to randomization as confirmed by independent radiologic review. Patients may have received 0 or 1 prior VEGF/VEGFR-targeted therapies. Patients were randomized to receive LENVIMA 24 mg once daily (n=261) or placebo (n=131) until disease progression. Patients in the placebo arm could receive lenvatinib following independent review confirmation of disease progression. The primary endpoint was PFS, as determined by blinded independent radiologic review using Response Evaluation Criteria in Tumors (RECIST) version 1.1. Secondary endpoints included objective response rate and overall survival.1,2
• PFS: 107 events (41%) occurred in the LENVIMA arm vs 113 events (86%) in the placebo arm1
– 93 patients (36%) who received LENVIMA progressed vs 109 patients (83%) who received placebo
– Death occurred in 14 patients (5%) who received LENVIMA vs 4 patients (3%) who received placebo
1L=first line; RAI-R=radioactive iodine-refractory; DTC=differentiated thyroid cancer; PFS=progression-free survival; CI=confidence interval; NE=not estimable; HR=hazard ratio; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid; VEGF=vascular endothelial growth factor; VEGFR=vascular endothelial growth factor receptor.
SECONDARY ENDPOINT
Superior response
65% ORRa with LENVIMA vs 2% ORR with placebo1,2
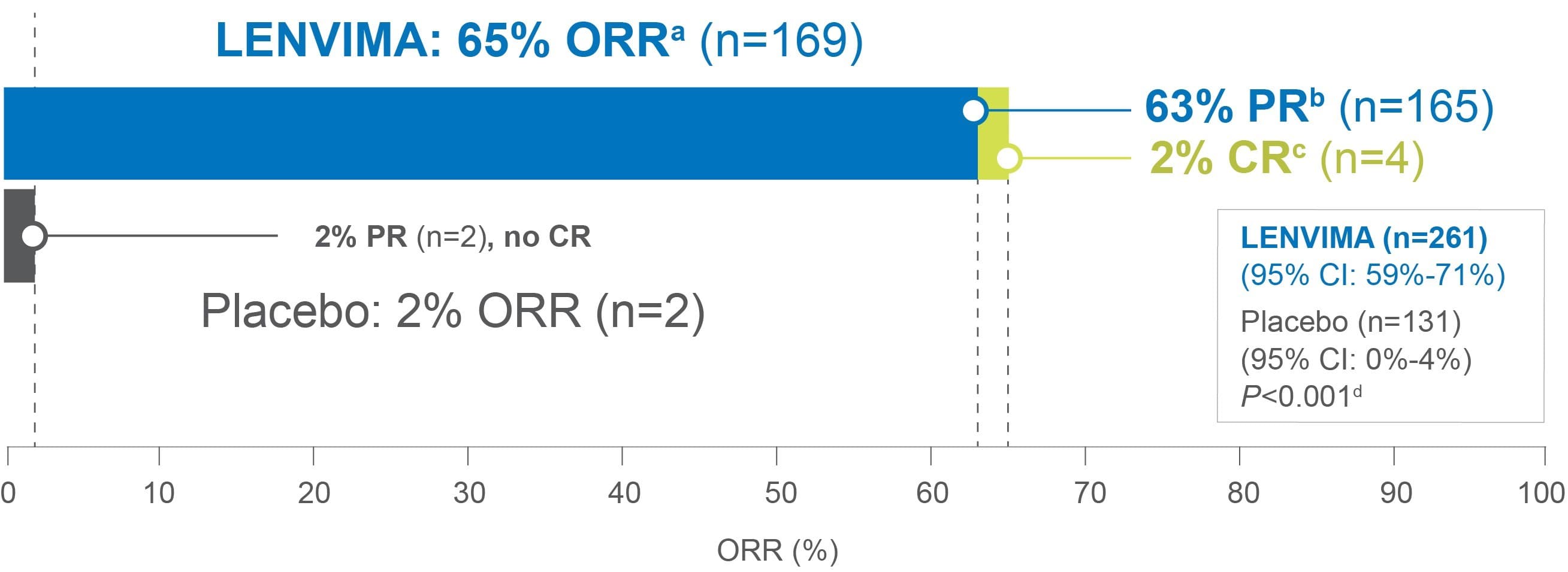
• First TKI to demonstrate a complete response in a phase 3 trial for locally recurrent or metastatic progressive RAI-DTC1-3
Overall survival (secondary endpoint)
• Median OS was not estimable at data cutoff (HR: 0.73 [95% Cl: 0.50-1.07]; P=0.10)1
–83% (109/131) of placebo-treated patients with confirmed disease progression crossed over to receive LENVIMA in the open-label extension phase (data cutoff: November 15, 2013)1,2
ORR=objective response rate; PR=partial response; CR=complete response; TKI=tyrosine kinase inhibitor; RAI-R=radioactive iodine-refractory; DTC=differentiated thyroid cancer; OS=overall survival; RECIST v1.1=Response Evaluation Criteria in Solid Tumors version 1.1.
aAssessed by independent radiologic review according to RECIST v1.1; Objective response rate (ORR)=sum of CR and PR.1,2,4
bPartial response (PR)=30% or greater decrease in the sum of diameters of target lesions.4
cComplete response (CR)=disappearance of all target and non-target lesions.4
dAccording to the Cochran-Mantel-Haenszel chi-square test.1

