Post hoc data in the SELECT trial
Duration of response analysis
30-month (95% CI: 18.4-36.7) median duration of response among patients who responded to LENVIMA1
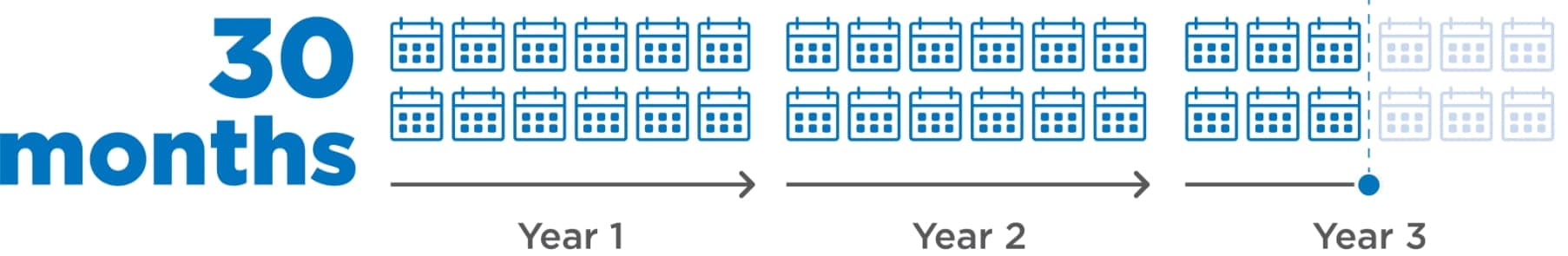
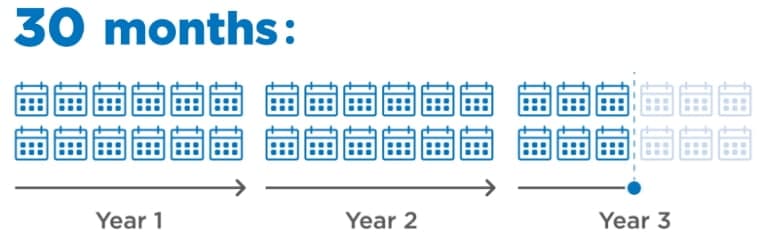
- Post hoc analysis (n=261) was conducted based on investigator-assessed response; 157 patients (60.2%) in the LENVIMA arm responded per investigator assessment1
Limitations: The post hoc exploratory subgroup analysis (data cutoff: September 1, 2016) was not a prespecified study endpoint. Patients who did not respond were not evaluated. No conclusions can be drawn.
CI=confidence interval; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid.
Median time to first objective response2


2.0-month (95% Cl: 1.9-3.5) median time to first objective response among patients who responded in the LENVIMA arm and 5.6 months (95% Cl: 1.8-9.4) among patients who responded in the placebo arm2:
- Analysis was conducted based on independent radiologic review of response: 169 patients (65%) in the LENVIMA arm (n=261) and 2 patients (2%) in the placebo arm (n=131) responded to treatment
- Tumor assessments using RECIST version 1.1 were performed every 8 weeks following randomization
Limitations: The exploratory subgroup analysis (data cutoff: November 15, 2013) was not a prespecified study endpoint. The outcomes of this analysis cannot be compared across treatment groups. Patients who did not respond were not evaluated. No conclusions can be drawn.
CI=confidence interval; RECIST v1.1=Response Evaluation
Criteria in Solid Tumors version 1.1.
PFS by site of metastasis3
Limitations: Exploratory analysis was not statistically powered to identify subgroup effects nor a multiplicity adjustment made. No conclusions can be drawn.
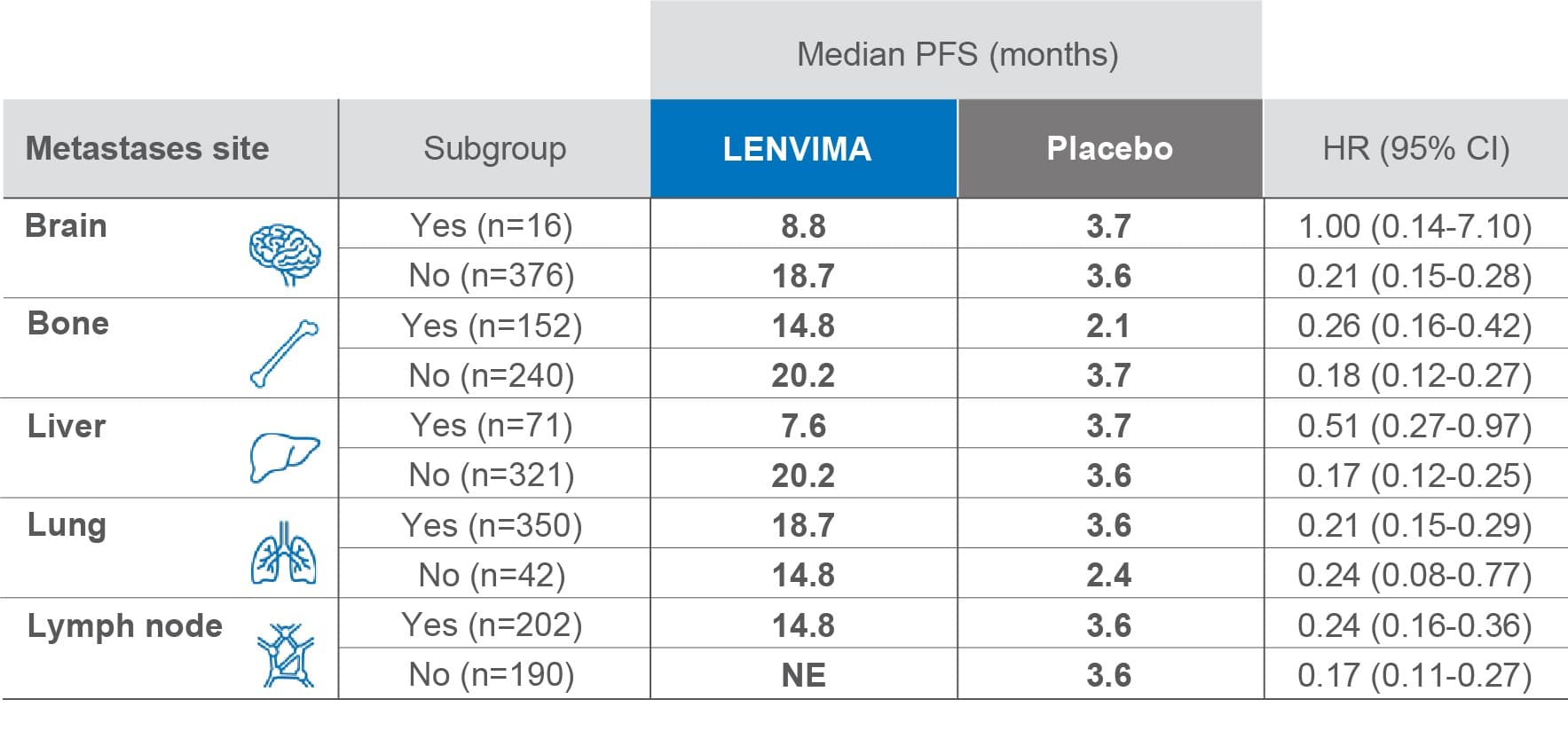
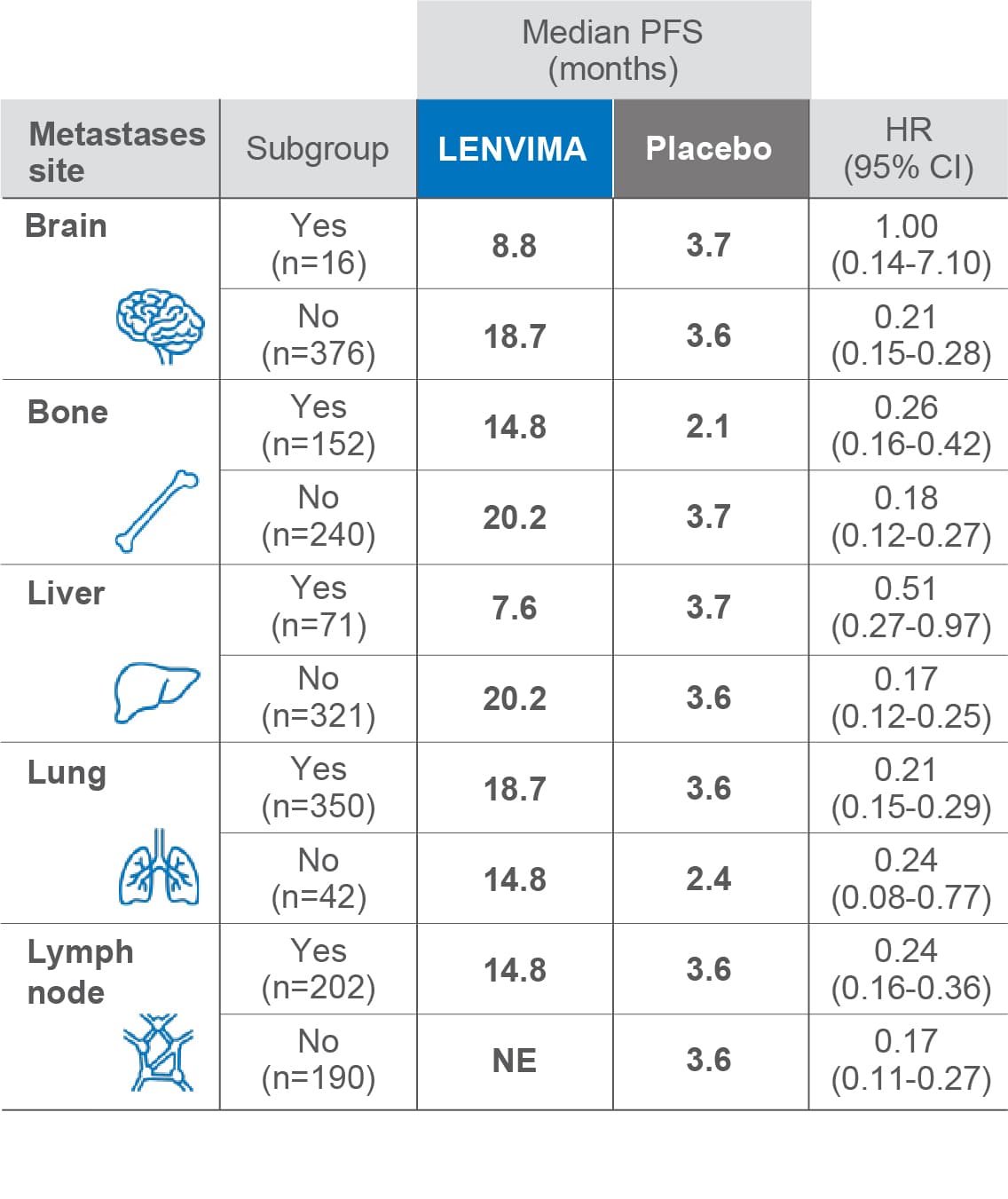
• The primary endpoint was PFS as determined by blinded independent radiologic review using RECIST v1.13
-
–This subgroup analysis examined patient efficacy outcomes based on baseline metastasis site
-
–Of 392 patients in the SELECT trial, 388 (99.0%) had ≥1 metastatic site
PFS=progression-free survival; HR=hazard ratio; CI=confidence interval; NE=not estimable; RECIST v1.1=Response Evaluation Criteria in Solid Tumors version 1.1; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid.
PFS in patients with baseline lung metastases ≥1.0 cm in SELECT3
Median PFS: 20.2 months with LENVIMA vs 3.7 months with placebo3
Limitations: Exploratory analysis was not statistically powered to identify subgroup effects nor a multiplicity adjustment made. No conclusions can be drawn.
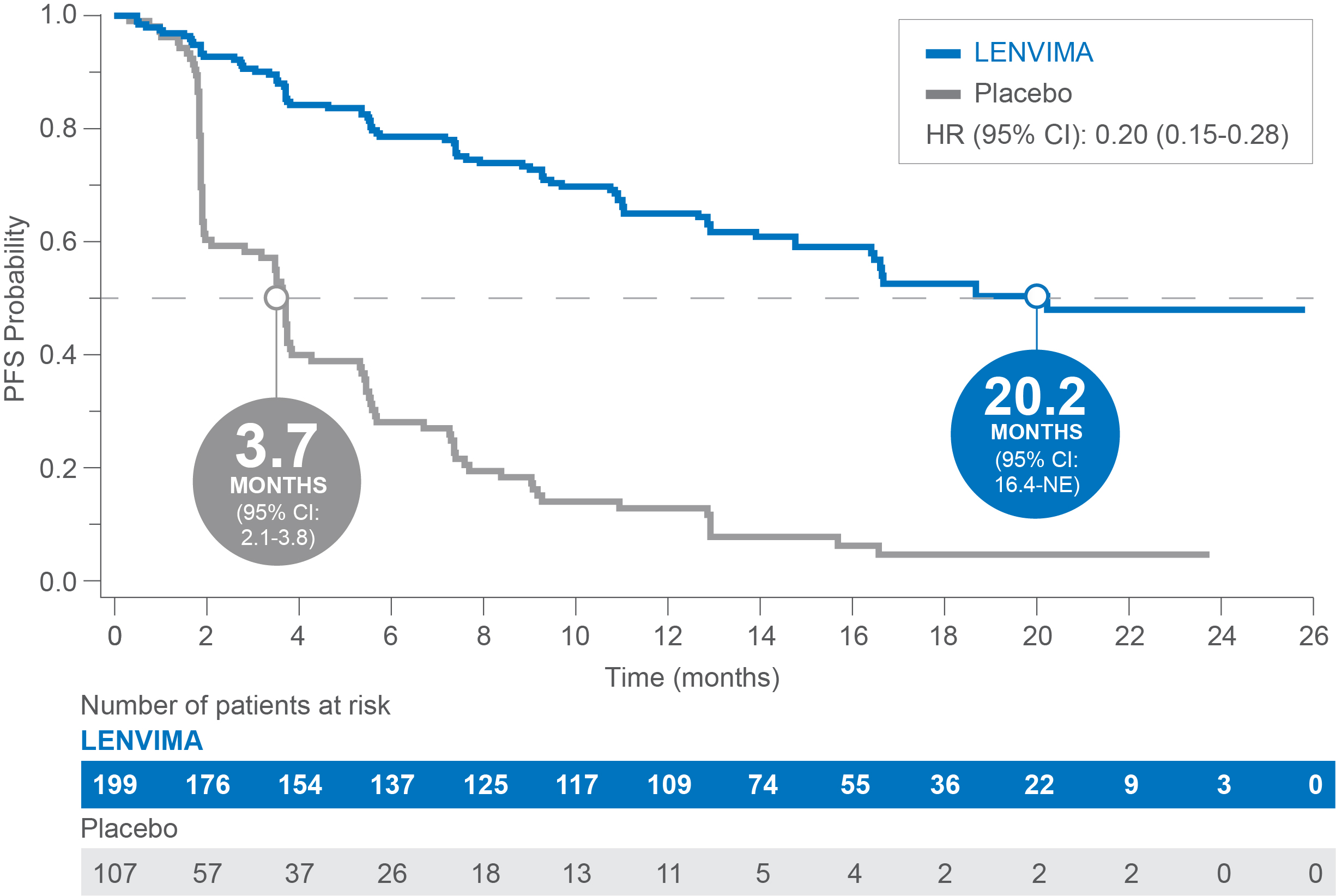

PFS=progression-free survival; SELECT= Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid; HR=hazard ratio; CI=confidence interval.
OS in patients with baseline lung metastases of any size in SELECT4
Median OS: 43.2 months with LENVIMA vs 34.0 months with placebo4
Limitations: Exploratory analysis was not statistically powered to identify subgroup effects nor a multiplicity adjustment made. No conclusions can be drawn.
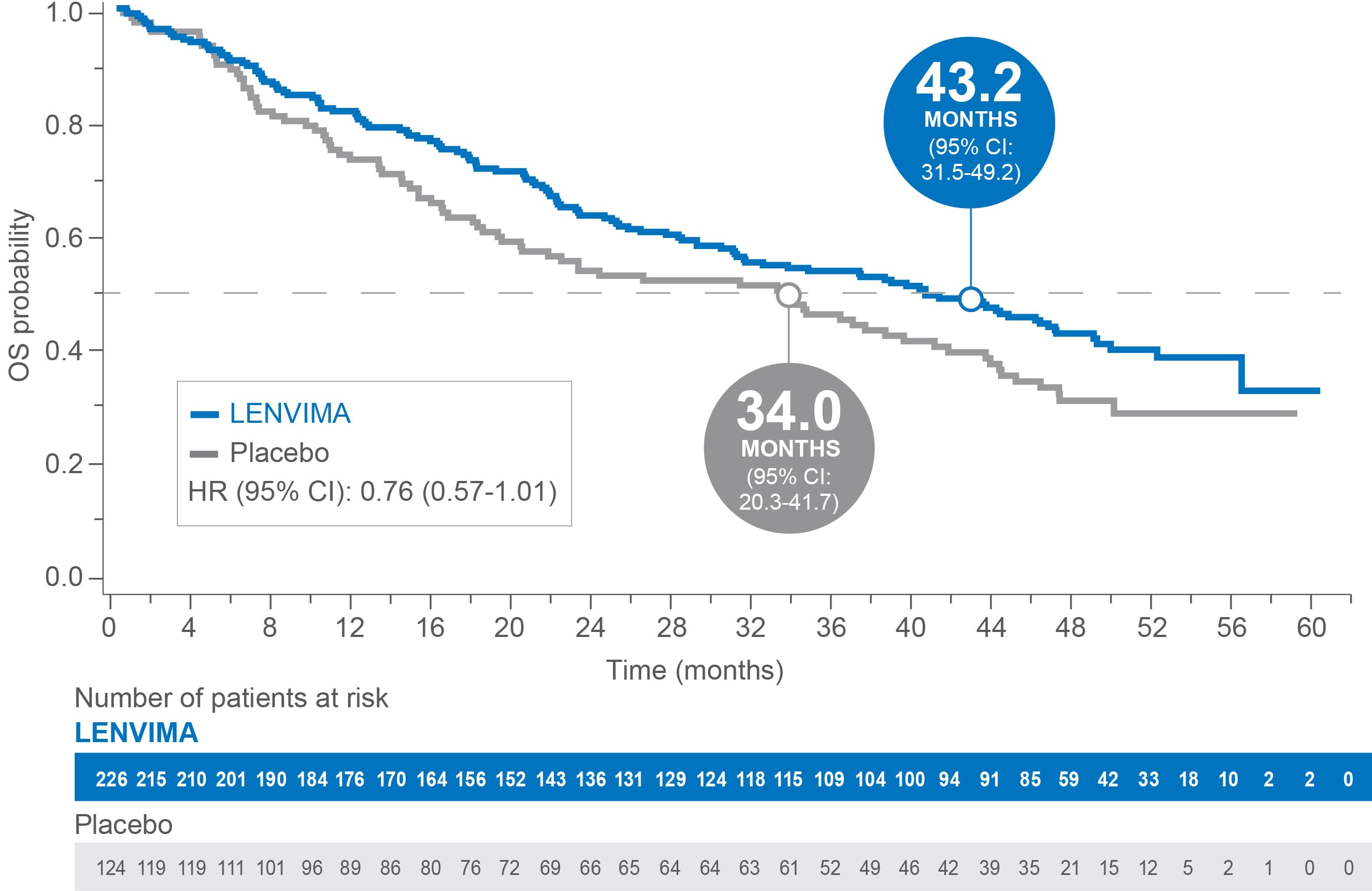
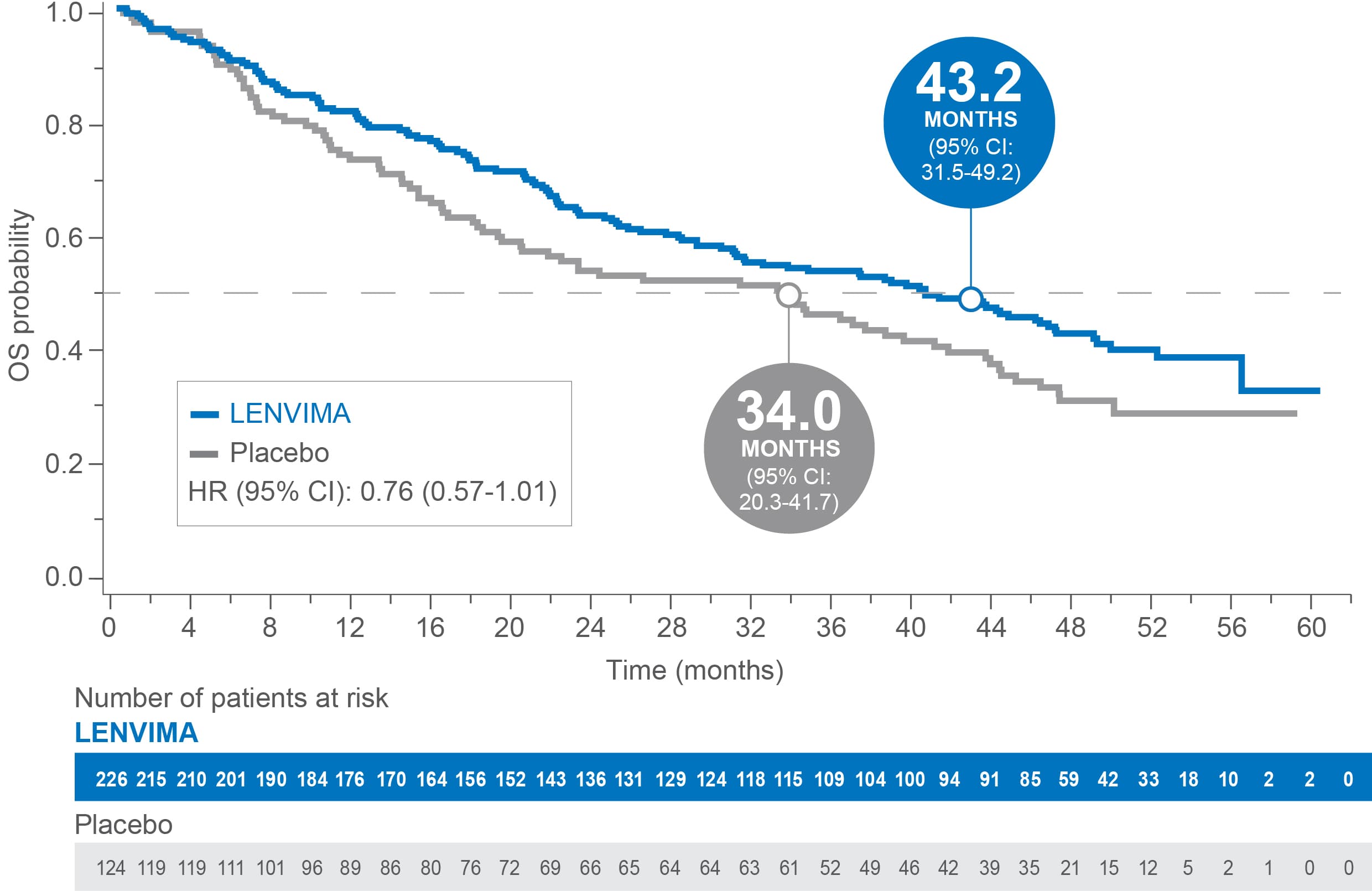
• Patients in SELECT were grouped by size of baseline lung metastases (≥1.0 cm, ≥1.5 cm, 2.0 cm, 1.0-2.0 cm) based on the criterion of a measurable lesion (≥1.0 cm) in RECIST v1.1. Efficacy and safety outcomes were assessed per lung metastases group
• In patients with any size of lung metastases, no significant difference in OS was observed between the LENVIMA and placebo treatment groups
OS=overall survival; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid; CI=confidence interval; HR=hazard ratio; RECIST v1.1=Response Evaluation Criteria in
Solid Tumors version 1.1.
OS in patients with baseline lung metastases ≥1.0 cm in SELECT4
Median OS: 44.7 months with LENVIMA vs 33.1 months with placebo4
Limitations: Exploratory analysis was not statistically powered to identify subgroup effects nor a multiplicity adjustment made. No conclusions can be drawn.
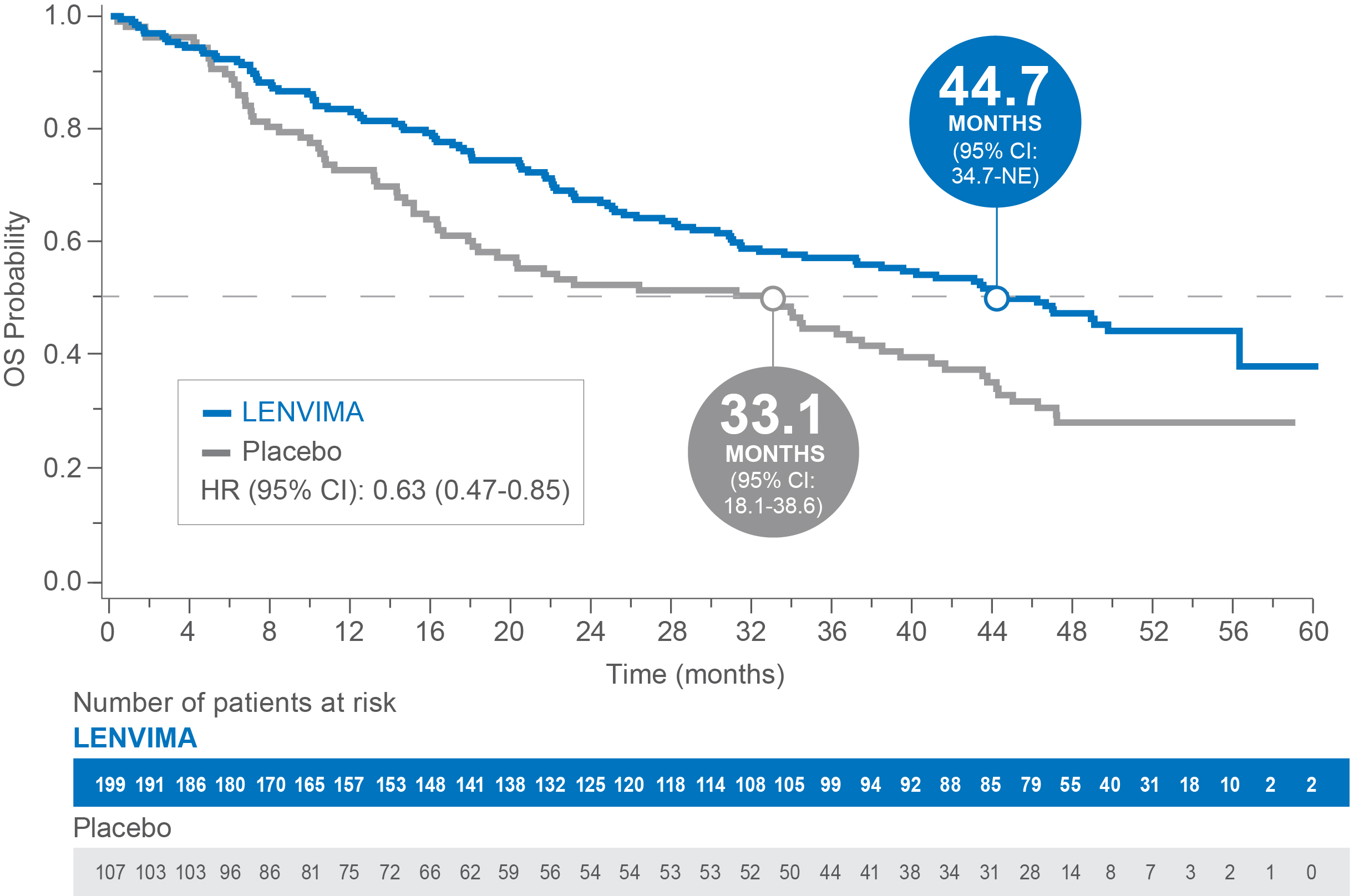
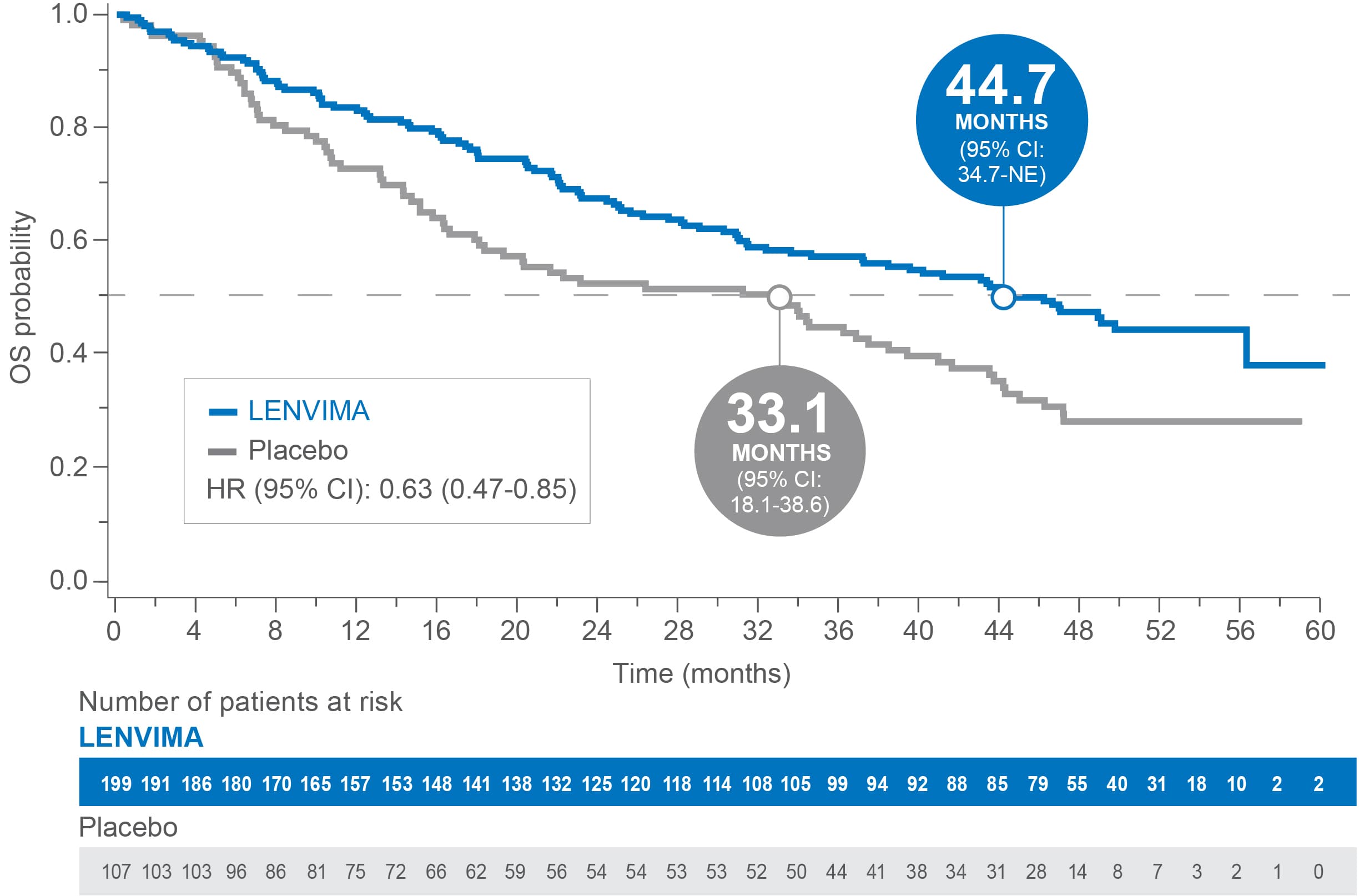
OS=overall survival; CI=confidence interval; NE=not estimable; HR=hazard ratio.
